Work packages
Overview
The TreatPreg project is a comprehensive research initiative focused on improving maternal and neonatal health in African regions heavily impacted by schistosomiasis, helminthiasis, and malaria. It features a Phase IV clinical trial to assess the safety and effectiveness of a new intervention, alongside pharmacokinetic studies and analysis of its socio-economic impact. The project also prioritizes stakeholder engagement, quality assurance, and building research capacity in Africa.
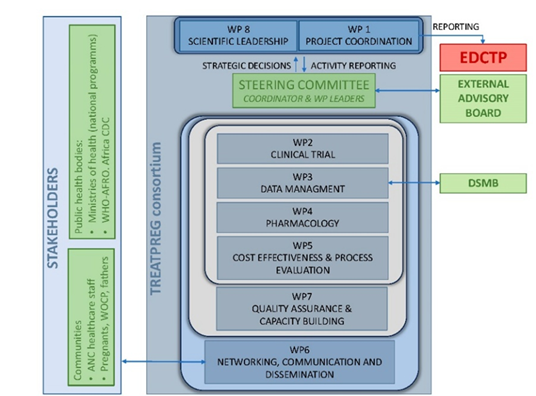
TreatPreg organigram

WP 1: Project coordination
This WP1 makes sure that the TreatPreg project runs smoothly from start to finish. It keeps all partners aligned, ensures the project stays on time and within budget, and guarantees that everything is done to the highest ethical and scientific standards.
What this includes:
- Strong coordination: Bringing together researchers, health experts, and stakeholders through regular meetings and clear communication.
- Good financial management: Tracking resources carefully to ensure transparency and accountability.
- Managing risks: Identifying possible challenges early and finding solutions to keep the project on track.
- Clinical trial support: Securing safe, high-quality medicines, insurance, and ethical approvals before starting the study.
- Reporting: Providing regular updates and results to funders, partners, and the wider public.
In short, WP1 is the backbone of TreatPreg — making sure that the project is well-organized, responsibly managed, and set up for success.
WP 2: The clinical trial
At the heart of TreatPreg is a large clinical trial across four African countries (Gabon, Ghana, Republic of Congo, and Benin). This study will test whether giving multiple antiparasitic medicines together during pregnancy is safe, effective, and practical to deliver through antenatal care.
What this includes:
- Running the trial: Around 2,400 pregnant women will take part through antenatal clinics. Some will receive the standard preventive medicines, while others will additionally receive praziquantel. Mothers and babies will then be followed until the child’s first birthday to carefully track safety and health outcomes.
- Studying the medicines in detail: In a smaller group of women, we will study how each medicine moves through the body during pregnancy, and how the medicines work when given together.
- Checking for infections: Women will be regularly tested for schistosomes, intestinal worms, and malaria parasites to measure how well the treatments prevent or reduce infections.
This WP2 ensures that the clinical trial is conducted safely, ethically, and to the highest international standards. The results will give clear answers about whether combining these treatments should become part of routine pregnancy care in Africa.


WP 3: Data management
For a study like TreatPreg, high-quality and reliable data are essential. This WP3 ensures that all information collected during the clinical trial is accurate, secure, and handled according to international standards.
What this includes:
- Building a secure database: A central system, accessible to all trial countries, will store the study data.
- Collecting information carefully: Doctors and nurses will record information about mothers and babies from the first antenatal visit until the child’s first birthday.
- Ensuring accuracy: Data will be checked, verified, and double-entered by trained staff to prevent mistakes.
- Keeping data safe: Strict safeguards, regular backups, and secure access will protect sensitive information.
- Preparing for analysis: Once all data are cleaned and verified, the final database will be locked and used for scientific and policy analysis.
In short, WP3 makes sure that the results of TreatPreg are built on trustworthy evidence, providing a solid foundation for improving pregnancy care in Africa.
WP 4: Studying how medicines work together
When several medicines are given at the same time, it’s important to know how they interact inside the body. This WP4 investigates whether the antiparasitic drugs used in TreatPreg affect each other’s safety or efficacy when combined during pregnancy.
What this includes:
- Understanding drug behaviour: We will study how each medicine is absorbed, distributed, and cleared from the body in pregnant women.
- Checking for interactions: By analysing blood samples, we will assess if taking the drugs together changes how well they work or increases side effects.
- Linking doses to outcomes: The study will explore how drug levels relate to infection clearance — for schistosomes, intestinal worms, and malaria parasites. This will help determine if current treatment doses are optimal for pregnant women.
- Building expertise in Africa: Local scientists will receive training in advanced pharmacology methods, including hands-on experience in Germany, to strengthen long-term research capacity.
In short, WP4 ensures that the combined use of these medicines is scientifically sound, safe, and effective for pregnant women and their babies.


WP 5: Costs, benefits, and real-life lessons
This WP5 looks beyond health outcomes to ask: Is adding praziquantel to routine antenatal care affordable, worthwhile, and practical?
What this includes:
- Value for money: We will calculate the extra costs of giving praziquantel during pregnancy, such as drug supply, staff training, and clinic time. We will also weigh these costs against savings from preventing complications like anaemia, preterm birth, or low birth weight.
- Wider impacts: The study will track how the treatment affects women’s quality of life, daily activities, and work, as well as their children’s early development and wellbeing.
- Understanding what works: By interviewing both mothers and healthcare workers, we will learn why the intervention succeeds or faces challenges — and what this means for rolling it out in real-life health systems.
- Decision tools for policymakers: A simple, easy-to-use model will show health authorities whether the benefits of adding praziquantel outweigh the costs, helping guide future policy.
In short, WP5 ensures that TreatPreg not only improves health but also makes economic and practical sense for healthcare systems.
WP 6: Building connections and sharing results
For TreatPreg to make a real difference, research findings must reach the people who can use them — from policymakers to local communities. WP6 focuses on building strong networks and sharing knowledge.
What this includes:
- Working with health authorities: Close collaboration with ministries of health in participating countries ensures that research aligns with national priorities and supports future maternal health guidelines.
- Engaging communities: Women, families, and healthcare workers (including midwives and doctors) are involved throughout the project. Their feedback helps shape the trial, while educational campaigns raise awareness about preventing parasitic infections during pregnancy.
- Making science accessible: Results will be translated into clear, easy-to-understand summaries with visuals and shared through community meetings, radio broadcasts, and clinics.
- Regional and global outreach: Findings will be shared with African and international partners such as WHO-AFRO, Africa CDC, and professional networks, ensuring broad visibility and uptake.
- Lasting impact: By feeding results into national and regional guideline development, WP6 helps ensure that successful strategies can be scaled up and sustained.
In short, WP6 connects science, policy, and communities to turn TreatPreg ’s evidence into action.


WP 7: Quality assurance and capacity building
This WP7 ensures that the TreatPreg study is carried out to the highest quality standards while also strengthening research capacity across all African partner sites.
What this includes:
- Strong quality standards: All laboratories and trial activities will follow international guidelines for clinical research. Regular training, audits, and monitoring will keep the project on track and ensure trustworthy results.
- Harmonized infrastructure: Equipment, procedures, and data systems will be standardized across all four trial countries so that results can be reliably compared and combined.
- Workshops and training: Short-term training sessions will cover topics such as good clinical practice, study design, data analysis, scientific writing, and community engagement. Materials and recordings will be shared on the project website for wider access.
- Supporting young researchers: MSc and PhD students from African partner institutions will be directly involved in the project. This hands-on experience will help build the next generation of experts in maternal health, pharmacology, and health economics.
- Lasting impact: By investing in people, infrastructure, and knowledge exchange, WP7 ensures that the benefits of TreatPreg continue well beyond the life of the project.
In short, WP7 combines quality assurance with capacity building, making sure the study is both scientifically rigorous and a driver of long-term growth in health research across West and Central Africa.
WP 8: Scientific project leadership
This WP8 is about ensuring that the scientific heart of TreatPreg stays strong, focused, and impactful. It provides leadership for the clinical trial and creates opportunities for young scientists to grow into future leaders.
What this includes:
- Training future leaders: A young PhD researcher will be trained through a “sandwich model” that combines local work at CERMEL with international mentoring and training. This hands-on experience will cover everything from clinical trial design and diagnostics to scientific writing and leadership skills.
In short, WP8 ensures that TreatPreg not only delivers excellent science but also builds future leadership in maternal health research in Africa.

